Complete On-Site Medical Research Testing Solution
Powered by TMS Pro4 POCT Molecular Diagnosis Analyzers
The TMS Pro4 POCT Molecular Diagnosis Analyzer, now CE-IVDR certified in the European Union, provides a fully integrated and compliant solution for rapid, accurate, and convenient molecular diagnostics at the point of care or research.

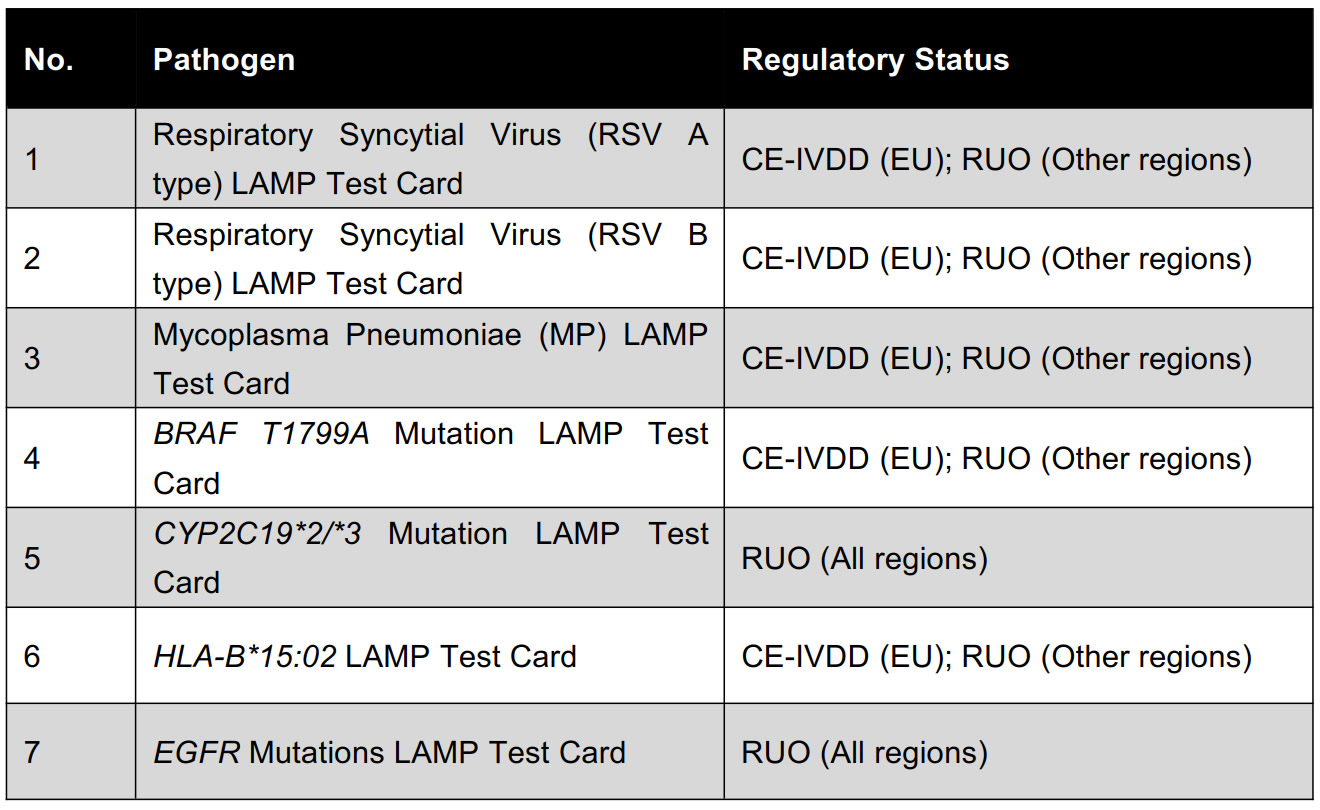
Paired with dedicated Test Card Kits—RSV A/B, Mycoplasma pneumoniae, HLA-B*15:02, and BRAF, all CE-IVDD certified—this system supports both regulatory-approved use in the EU and research-use-only (RUO) in other markets. It is ideal for laboratories, hospitals, clinical trials, and academic research settings.
✔ Fully automated testing workflow
✔ Minimal sample preparation required (e.g., swabs, extracted DNA)
✔ Results in 30–40 minutes
✔ Covers infectious pathogens and genetic mutations
✔ Suitable for infectious disease research, drug safety screening, and personalized medicine
✔ Certified for clinical diagnostic use in the EU

With the TMS Pro4 system, you can:
• Conduct molecular diagnostics in compliance with EU IVDR standards
• Detect critical respiratory and genetic targets with clinical-grade accuracy
• Improve research efficiency and accelerate data-driven discoveries
• Enable safe and effective deployment in both research and clinical environments
Accelerate your research and clinical diagnostics — with speed, confidence, and compliance.
TMS Pro4: Trusted molecular insight, wherever it’s needed.
Device:

CE-IVDR (EU); RUO (Other regions)
Test Target List: